
A closer look at the concept of temperature
A closer look at the concept of temperature
– by Peter Koelewijn, 24-09-2018
Temperature may be the most familiar property of HVAC but it is also, the most elusive. We often think of it in such common terms – setting the temperature on our thermostat to a comfortable level; the weather forecast shows us the temperature today – that it can be easy to forget how complex the subject really is.
So what is the definition of temperature? Yes, it is a reference to how warm or cold something is. But what exactly are we saying when we talk about hot or cold? Let me take you on a thought experiment in which we pretend we’ve never heard of the word ‘temperature’.
An experiment
In thermodynamics, a ‘system’ is a region containing energy and/or matter that is the subject of an investigation or analysis. Everything external to the system is the ‘surroundings’. Our experiment assumes two closed systems shaped as cylinders, each containing a movable piston. The cylinders are connected to each other by a ridged rod attached to the pistons.
When the piston moves rightward, the pressure in the right cylinder increases with regard to that in the left cylinder. We can say this even without measuring the pressure simply by observing the position of the piston.
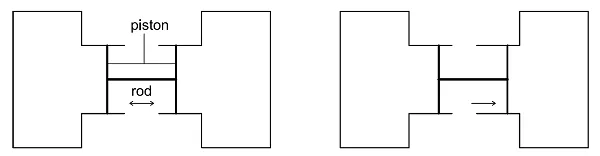
Cross-section of two cylinders
The equaliser
If the piston is in a neutral position – the so-called equilibrium state – the pressure is the same in system A and system B.
Now let us assume that there is a third separate cylinder, system C. If we connect system A to system C and the pistons don’t move, the systems are in mechanical equilibrium. We see that the pressure in system A is the same as in system C, which means that the pressure in system B is the same as that in system C – we can conclude this without connecting them.
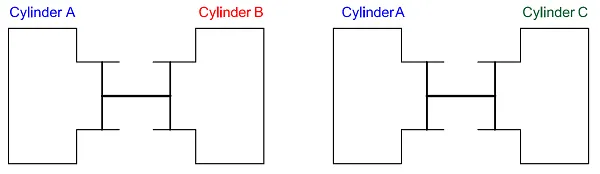
Two cylinders in equilibrium
Zeroth law of thermodynamics
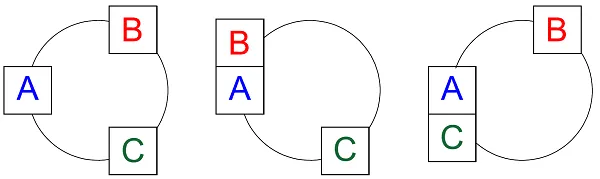
Let’s move from mechanics to thermodynamics. Suppose systems A and B have rigid walls – no pistons – and are brought in contact with each other. If they are at different temperatures, heat will flow between them. Once they have reached a point where such a flow no longer occurs, the two systems can be said to be in thermal equilibrium. The same will then apply to the relationship between systems A and C as did with the pistons. This is an illustration of the zeroth law of thermodynamics:
"If A is in thermal equilibrium with B, and A is in thermal equilibrium
with C, then B will be in thermal equilibrium with C."
Pressure is a physical property that lets us measure whether systems are in a mechanical equilibrium, no matter their size or design. As explained by the zeroth law of thermodynamics, temperature can be used to measure thermal equilibrium, no matter the size or composition.
The definition of a thermometer
This might sound obvious, but it is exactly how a basic thermometer works. It makes use of the thermal expansion of mercury or another expanding liquid. If we then bring the thermometer (system A) into contact with – for example – a pipe in front of a heat exchanger (system B), the temperature in the two systems will eventually equalise. If we then bring the thermometer in contact with the pipe after the heat exchanger (system C) and measure the changes, we can tell the temperature difference before and after the heat exchanger.
While temperature and pressure are different properties, they are close connected to each other. But what is the link between the two? The answer is in the energy state of molecules.
The concept of a thermometer
Excitement on a molecular level
Everything on earth is built out of molecules: your clothes, your body, the water in your pipes at home and the wind in the sails of a yacht. The temperature of those molecules indicates their energy state: the higher it is, the more excited it is said to be.
The brilliant Austrian physicist Ludwig Boltzmann came up with a way to measure the energy state of molecules. He formulated the energy level of a given set of molecules in equilibrium (as in our experiment) as a single parameter, β (beta). The definition of temperature is the average speed of molecules in a system. High temperatures correspond with high average speeds and low temperatures to lower average speeds.
Difference between Fahrenheit and Celsius
While parameter β is the most accurate measure of temperature, before it existed several clever people already defined the concept of temperature and found a way to measure it.
For instance, the Swedish astronomer Anders Celsius invented a scale on which water froze at 100° and boiled at 0° (you didn’t misread, it was the opposite of the Celsius scale we use today). The German instrument maker Daniel Fahrenheit instead came up with a scale where 0° was the lowest temperature he could achieve (using a mixture of salt, ice and water). He set his own body temperature at 100°.

Fahrenheit & Celsius scales
The reality of cold
These scales have come to define our understanding of temperature. When we talk about hot and cold, we automatically imagine them in terms of the Celsius or Fahrenheit scale in our minds. We say that things are boiling hot or freezing cold.
While this suffices as these are the ranges we are used to in everyday life, for the purposes of physics there is no such thing as cold – things can only be more or less warm – and the term ‘cold’ is merely a convention of language.
The lowest possible temperature
Let’s put on our science goggles and look at the concept of temperature as a physicist. We have seen that temperature reflects the energy level of molecules, with which temperature rises and falls.
The lowest temperature reflects a point where all the molecules stand still. This is called the ‘ground state’. The scale most commonly used in science, the Kelvin scale (named after the Scots-Irish mathematical physicist and engineer William Thomson, the first Baron Kelvin), takes this as its zero degrees.

In the ground state, every atom is frozen
Chaos and turmoil
As a conclusion, we can say the following. Looking from the outside, temperature can be defined as a property that shows us whether two closed systems that are in contact (think of the thermometer) are in thermal equilibrium or not (over time the situation will tend toward equilibrium). From the inside, at a sub-molecular level, temperature designates the energy that causes atoms to move, spin and vibrate. In other words, temperature is a measure of molecular turmoil.
Peter Koelewijn | Sales Manager
Peter Koelewijn has been working at Heinen & Hopman since 2001. Starting out as a mechanic, he gained valuable field experience mounting ducts and pipes for various shipbuilding projects. Five years later he switched to engineering, where he worked his way up from draughtsman, to engineer to site manager, leading teams of HVAC mechanics at one of the largest shipyards in Germany. The last couple of years he has been working as sales manager, combining knowledge from the field and the office to find the best solutions for our customers.
Source: This blog was based on Peter Atkins’ book: Four laws that drive the universe
Pictures: Pexels, Pixabay