
What is entropy? Part 2: Birth of the Second Law
What is entropy? Part 2: Birth of the Second Law
– by Paul Spoorenberg – 07/08/20
Ever wondered why heat never flows from cold to hot? Why a machine with 100% efficiency can never be achieved and why we know our past but not our future? It all has to do with a concept called Entropy.
In this blog we dive into the history of entropy and see how the German physicist Rudolph Clausius eventually came up with the concept of entropy and the Second Law.
Part one: a simple definition
Part two: birth of the Second Law
Part three: the way the universe will end
Ideas from a Victorian era
Like every animal on earth, man uses energy. When we were hunter-gatherers our primary energy sources where animals and plants. Later we discovered the power of wind (sailing boats and mills), whale oil (lamp oil) and peat and coal (excellent heat sources).
But the big change came with the industrial revolution early eighteenth century. Someone got the idea to bring water to a boil over burning coals. This created steam and with that steam he set a pump or turbine in motion and with that movement he let other things move.
Connect a steam engine to a loom, for example, you can replace the work of tens of human hands by burning a large container of coal. Eureka!
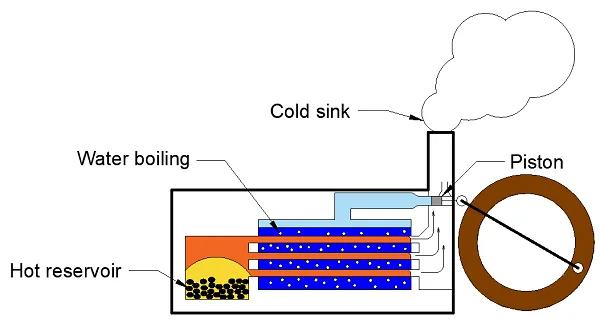
Steam engine
Suddenly, the steam engine entered daily life and changed the social and economic structure. Just as the computer and social media would do a century later. England was the Silicon Valley of the time.
The French looked enviously at the thick plumes of smoke rising from the other side of the Channel. In France, there was a lack of accessible coal. Therefore, the urgent task of the French to improve the efficiency of steam engines. More work for less coal.
Sadi Carnot
It all began with Sadi Carnot. Born in 1796 in Paris, he was the third and final Sadi, the two earlier children with the same name having already passed away. Sadly, cholera would take the life of this Sadi too at 36 years of age, but not before he had made himself immortal with a remarkable contribution to science.
Carnot considered heat to be a fluid which flowed from the heat source (burning coals) to the cold sink (exhaust pipe). Based on this model he came up with a theory to determine the efficiency of an idealised steam engine. We still use this method today to determine the efficiency of an ideal cooling machine or heat pump, as you can read here.
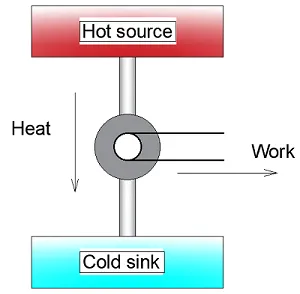
Diagram representing a steam engine
Lord Kelvin
A little over half a century later William Thomson – or Lord Kelvin as we know him better – built further on the theories of Sadi Carnot. His conclusion was as obvious as it is brilliant.
Imagine standing in front of a nineteenth-century steam engine. What will be its most essential component? The piston or the hot reservoir maybe?
No, the key component is the cold sink. In other words, the surroundings in which the waste heat is discarded. Take away the cold sink and the engine stops running even though plenty of fuel is available and all the gears and pistons are perfectly lubricated and greased.

The Kelvin statement
Kelvins statement asserts that no (steam) engine will work without a cold sink into which some heat must be wasted.
Rudolph Clausius
Around the same time the German physicist Rudolph Clausius made a discovery that is also in hindsight pretty obvious. He saw that heat never spontaneously flows from a cooler to a hotter body. By spontaneous, Clausius meant natural, without the help of a heat pump or refrigerator.
In fact, the statements of Kelvin and Clausius are both sides of the same medal. Clausius was aware of this and searched for a way to bring them together so he could apply this theory to all phenomena in the universe.
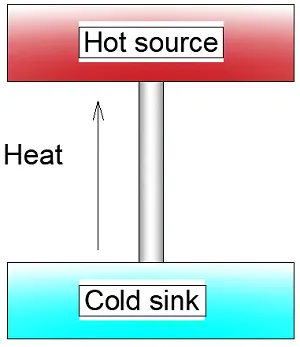
The Clausius statement
The introduction of entropy
In 1856 Clausius first introduced the concept of entropy, defining it as the change in entropy that occurs when energy is transferred to a system as heat. Remember the equation from our last blog.
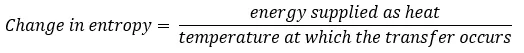
Note that the change in entropy is given by the energy transferred as heat and not by energy transferred as work and remember that entropy never decreases.
Now, let’s see what happens as we bring both statements in line with the concept of entropy.
First, take Kelvin’s statement that an engine will only work with a cold sink. Heat is removed from the hot source, so the entropy of the reservoir goes down. If all energy is transferred as work and there is no energy (heat) wasted, entropy would not increase.
A change in entropy only occurs when energy is transferred as heat, not as work. So, without the cold sink entropy decreases and according to Clausius that is impossible, so the Kelvin statement is true.
Now let’s turn to Clausius’ own statement that heat always flows from hot to cold.
Let’s say we have a glass of cold water and put it in a hot oven. The heat out of the glass will never flow to the oven: it cannot because the change in entropy at a low temperature will be large as temperature occurs in the denominator of the equation. If that same heat energy extracted from the cold glass enters the interior of the oven, the change in entropy is small.
The net effect is that the entropy decreases and, as we have seen, that is impossible. So heat can never flow spontaneously from cold to hot.
Birth of the Second Law
There you have it. Clausius’ introduction of entropy neatly captures the empirical laws that bound together the Second Law of thermodynamics. It concerns all spontaneous processes that happen in the universe. You can apply it to the expansion of gases or predict in which direction a chemical process will run.
There has never been an exception to the Second Law.
But what does this mean this entropy and its inability to decrease? That will be the topic of our final blog about entropy.
Paul Spoorenberg | Sales Manager
Paul Spoorenberg has been working at H&H since 1994. During his career he gained valuable expertise in HVAC solutions for all kind of vessels in the Commercial, Offshore, Naval & Yacht segments.